For more information about UMF:
- How to Calculate the Unity Molecular Formula by Ceramic Materials Workshop https://www.youtube.com/watch?v=HyLjAg1_8_4
- Linda Arbuckle’s Introduction to Glaze Calculation
- How Glazes Melt by Dave Finkelnburg
- Digitalfire’s Understanding Glaze Calculation
- Linda Bloomfield’s Colour in Glazes has a section on UMF and the Stull Chart.
UMF in Glazy
Glazy currently has two types of UMF calculation: original/traditional as well as “extended”.
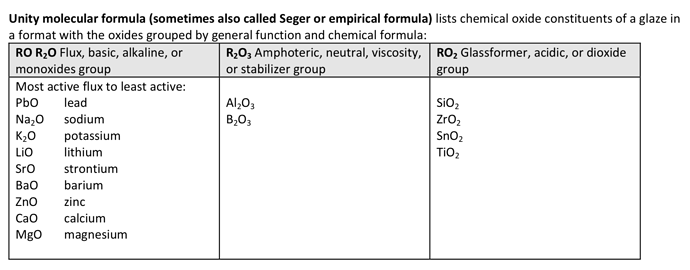
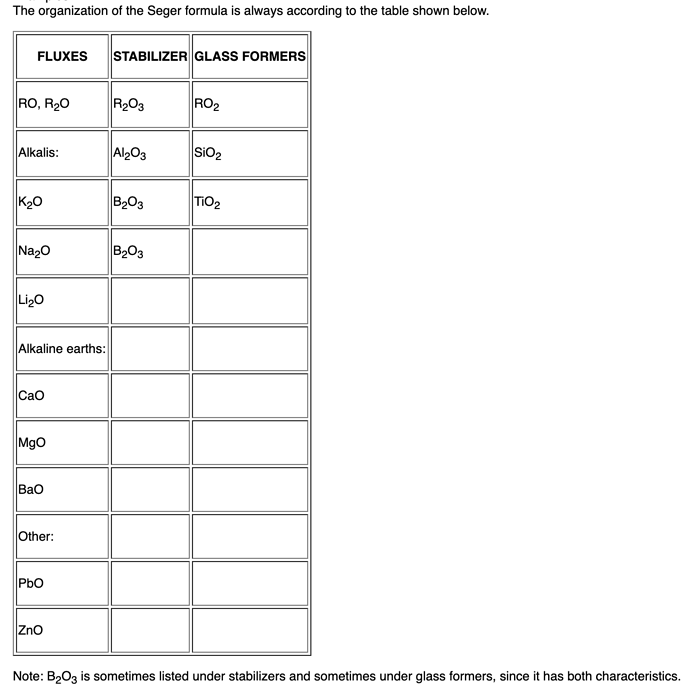
The “traditional” UMF adheres to the oxide groupings of RO/R2O, R2O3, and RO2, where RO/R2O are used for unity. Any oxides not listed in those groups are simply listed as “OTHER”.
The “extended” UMF attempts to define roles for a broader range of oxides, for example CuO and CoO, that were previously viewed as secondary (e.g. “colorants”) and classified outside the unity as “other”. This version of the UMF is still a work in progress. It is based in part on the work of Matt Katz and Rose Katz. (See Rose Katz’s 2019 NCECA Lecture “COLORFORMS” which discusses the roles of all major colorants.)
Glazy “Traditional” UMF Oxides
RO_R2O: Na2O, K2O, Li2O, PbO, SrO, BaO, ZnO, CaO, MgO, MnO
R2O3: Al2O3, B2O3
RO2_OXIDES: SiO2, ZrO2, SnO2, TiO2
Glazy “Extended” UMF Oxides
RO_R2O: Na2O, K2O, Li2O, PbO, SrO, BaO, ZnO, CaO, MgO, MnO, Bi2O3, CuO, SnO2, Fe2O3, MnO2, CoO
R2O3: Al2O3, B2O3, TiO2, NiO
RO2: SiO2, ZrO2
“Extended” UMF Origins
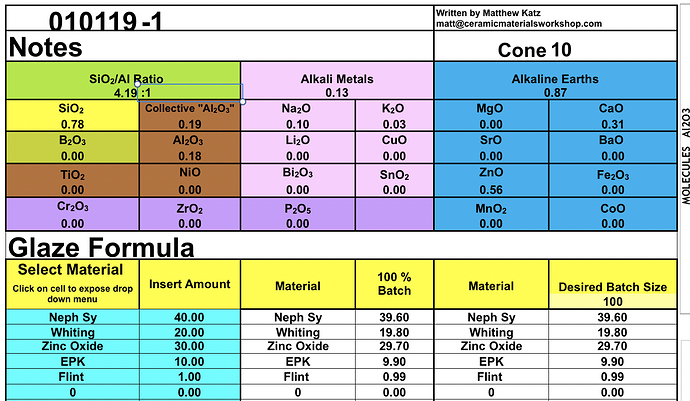
Glazy’s “Extended” UMF comes directly from Matthew & Rose Katz’s Experimental UMF Calculator. This spreadsheet calculator can be downloaded here.
Screenshot of Calculator:
This Experimental Calculator is still a work in progress. Glazy will be updated as new information comes to light.
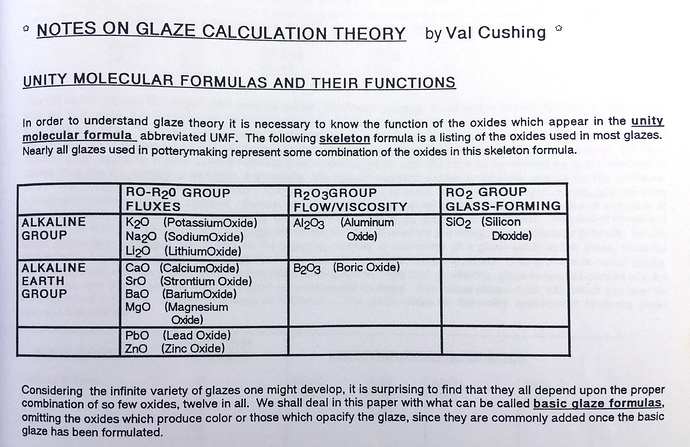
Oxides Used in Historical UMF Calculation
Daniel Rhodes “Clay and Glazes for the Potter”:
Val Cushing’s Handbook:
Linda Arbuckle’s Glaze Calc Handout
https://lindaarbuckle.com/handouts/glaze-calc-intro.pdf
Henrik Norsker, James Danisch: “Glazes for the Self-Reliant Potter”
http://collections.infocollections.org/ukedu/en/d/Jg17gle/17.2.html